FAQ
Frequently asked questions on W&H products and helpful descriptions on the topic of hygiene and maintenance.

 Couplings
Couplings

-
Roto Quick (5)
-
Can all instruments that I was able to use with the old Roto Quick coupling models (924 etc) also be attached to the current Roto Quick models (RQ-24 etc)?
Yes. All existing and new instruments can be used with couplings from both the old and the new generations.
-
How does the new W&H Click & Pull system work? How do I remove a turbine?
Use your thumb and index finger to retract the coupling's retention sleeve. The turbine can now be removed from the coupling without any problems. Pulling forcefully on the dental turbine can cause damage. If excessive force is required to remove the turbine, verify that the retention sleeve on the coupling can be moved without any problems.
-
Why do I need a 6-hole connection to operate a standard LED turbine?
A 6-hole connection is necessary to supply the turbine with electricity. Other connections do not provide a power supply. The only turbines that do not require a 6-hole connection are the self-generating Alegra LED G turbines which can also be used on non-optic connections.
-
Can the W&H coupling be thermo washer disinfected and sterilized?
Thermo washer disinfection of the coupling is not permitted; however, current models of W&H coupling can be sterilized in steam sterilizers using a temperature of 135°C. Hot air sterilization and/or cold sterilization (placing in liquids) are not approved techniques.

-
Are there other couplings than the Roto Quick available?
Yes. W&H also offers the "RM-34 LED" for MULTIflex® connection.
-
Can all instruments that I was able to use with the old Roto Quick coupling models (924 etc) also be attached to the current Roto Quick models (RQ-24 etc)?
 Accessories
Accessories
-
Piezo Scaler Tips (5)
-
Are W&H tips (e.g. 1U) compatible with the new W&H high-speed mounting system?
No, they are not compatible because they have different threads.
-
How do I know which tip goes with which handpiece?
There are symbols on the tip, the tip changer and the handpiece identifying which system they belong to.
-
Are the new handpieces compatible with the PA-123/PA-115?
No, they are not compatible.
-
Can the tips and tip changers be put through the sterilizer?
Yes, as long as they are indicated on the instructions for use with the mark “sterilizable up to the stated temperature”.
-
Are the tips and tip changers thermo washer disinfectable?
Yes, as long as this is indicated in the instructions for use and they carry the mark “thermo washer disinfectable”.
-
Are W&H tips (e.g. 1U) compatible with the new W&H high-speed mounting system?
-
Prophy Cups and Brushes (3)
-
Can I use the W&H Proxeo TWIST LatchShort prophy cups in a contra-angle handpiece with a standard 2.35-mm chuck?
Due to the shorter shaft length of the W&H prophy cups, they cannot be gripped by a standard contra-angle handpiece chuck , so it is not possible to drive the W&H prophy cup.
-
Can I use the Proxeo TWIST LatchShort Prophy cups and brushes multiple times?
No, the Proxeo TWIST Prophy cups and brushes are single-use only.
-
Can I use my LatchShort Prophy cups and brushes with other contra-angle handpieces?
No, LatchShort Prophy cups and brushes are designed to be used only with the WP-66 W contra-angle handpiece.
-
Can I use the W&H Proxeo TWIST LatchShort prophy cups in a contra-angle handpiece with a standard 2.35-mm chuck?
 Saw Handpieces
Saw Handpieces

-
How to dismantle the handpiece
See detailed description in user manual or Video.
 Implant stability measurement
Implant stability measurement

-
Osstell Classic (3)
-
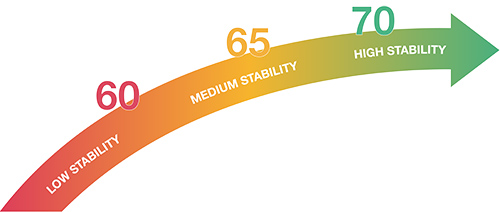
How does the measurement of the ISQ work?
> To measure the ISQ of an implant, you first screw a 'SmartPeg' into the placed implant. The SmartPeg, with its magnet on top, works like a small tuning fork. The magnet on the SmartPeg is ‘hit’ with magnetic pulses, from the probe, which makes the SmartPeg vibrate. Due to the stiffness in the interface between the implant surface and the bone the SmartPeg will vibrate accordingly. The more dense the bone is the higher stability and the higher ISQ value.
-
Where can I buy the SmartPegs?
Please contact your local W&H UK partner
A voucher will be included with any unit purchased, enabling the purchaser to request 5 free SmartPegs. -
What does ISQ stand for?
The abbreviation ISQ stands for Implant Stability Quotient. It is a value between 1 and 100 and gives the surgeon an insight of the implant stability after placing the implant. With the ISQ value the surgeon can monitor the osseointegration.
-
How does the measurement of the ISQ work?
-
Osstell Beacon (3)
-
How does the measurement of the ISQ work?
> To measure the ISQ of an implant, you first screw a 'SmartPeg' into the placed implant. The SmartPeg, with its magnet on top, works like a small tuning fork. The magnet on the SmartPeg is ‘hit’ with magnetic pulses, from the probe, which makes the SmartPeg vibrate. Due to the stiffness in the interface between the implant surface and the bone the SmartPeg will vibrate accordingly. The more dense the bone is the higher stability and the higher ISQ value.

-
Where can I buy the SmartPegs?
Please contact your local W&H UK partner
A voucher will be included with any unit purchased, enabling the purchaser to request 5 free SmartPegs. -
What does ISQ stand for?
The abbreviation ISQ stands for Implant Stability Quotient. It is a value between 1 and 100 and gives the surgeon an insight of the implant stability after placing the implant. With the ISQ value the surgeon can monitor the osseointegration.
-
How does the measurement of the ISQ work?
 Air Scaler
Air Scaler

-
Proxeo (1)
-
Can my W&H handpiece be sterilized?
Yes, providing that your W&H handpiece carries the relevant sterilization symbol.

-
Can my W&H handpiece be sterilized?
 Electric Motor
Electric Motor

-
Electric Motor (EM-12 L) (10)
-
How can I integrate the electric motor into my unit as a built-in version?
The electric motor can be integrated into a dental unit by selected dental unit manufacturers or their service technicians.
-
How do I connect the electric motor to my unit as an Add-on?
To connect the electric motor to your dental unit as an Add-on you require a free turbine hose on your dental unit and the possibility of connecting the control to the mains via the included power supply unit. You can control the electric motor using the foot control of your dental unit.
-
Can I sterilize my electric motor in a sterilizer?
Yes, if your electric motor features the symbol “Sterilizable up to the stated temperature”.

-
Can my electric motor be reprocessed in a thermo washer disinfector?
No.
-
Does the Built-in version of the electric motor require maintenance?
The Built-in electronic component does not require separate maintenance. However, regular servicing together with the rest of the dental unit should be performed at the intervals prescribed by the pertinent legislation. This makes it possible to guarantee proper function and safety. More detailed information on this topic can be found in the “Service” section of the respective instructions for use.
-
Does the Add-on version of the electric motor require maintenance?
The electric motor as an Add-on version including accessories requires a regular check once every three years unless shorter intervals are prescribed by law. The regular service must only be performed by an authorised W&H service partner. More detailed information on this topic can be found in the “Service” section of the instructions for use.
-
Does the electric motor need to be lubricated with oil?
No, the motor bearings require no maintenance. Any additional oil lubrication would reduce the service life of the bearings.
-
Should I always call my W&H service partner in advance before sending devices/instruments in for repair?
If you are not completely sure of the cause of the problem, it is better to contact your W&H service partner first and speak to a technician. It is sometimes possible to resolve the problem over the phone otherwise the product will need to be sent in for repair.
-
What is a brushless electric motor and what benefits does it offer me as a user?
The brushless electric motor is considerably less susceptible to wear than a brush motor, which in turn translates to minimised service and maintenance work. This also means less downtime for the user when servicing proves necessary.
-
Which contra-angle handpiece series is recommended for the electric motor?
The W&H Synea series – the glass rod elements are optimally suited for use together with the motor and its integrated LED+.
-
How can I integrate the electric motor into my unit as a built-in version?
-
Electric Motor (EM-E6) (2)
-
I've got a micromotor without light. Do I have to change the micromotor and the contra-angle to have light?
No. You can purchase an Alegra LED G contra-angle which has its own light generator and LED outlet. No further investment is necessary.
-
Does the electric motor need to be lubricated?
No, the motor bearings require no maintenance. Any additional lubrication would reduce the service life of the bearings.
-
I've got a micromotor without light. Do I have to change the micromotor and the contra-angle to have light?
-
LED Upgrade Set (2)
-
I've got a micromotor without light. Do I have to change the micromotor and the contra-angle to have light?
No. You can purchase an Alegra LED G contra-angle which has its own light generator and LED outlet. No further investment is necessary.
-
Does the electric motor need to be lubricated?
No, the motor bearings require no maintenance. Any additional lubrication would reduce the service life of the bearings.
-
I've got a micromotor without light. Do I have to change the micromotor and the contra-angle to have light?
 Cleaning & Disinfection Agents
Cleaning & Disinfection Agents

-
BePro (5)
-
How do I choose the right disinfectant for my practice?
Depending on the categories of pathogenic microorganisms on which the disinfectant is effective, different levels of disinfection are distinguished; high, intermediate and low. In order to ensure total safety in the practice, for both patients and operators, the choice of disinfectant to be used must always fall on a product that ensures a high level of disinfection.
-
What are the main aspects I have to consider when choosing a disinfectant?
There are three fundamental aspects that must always be considered when choosing a disinfectant: the desired efficacy, i.e. the ability of the product to be active on a wide range of pathogens, the expected efficiency, i.e. the ability of the product to reach its maximum effectiveness in the shortest possible time and, in the case of concentrated products, with the lowest possible concentration and finally the necessary compatibility, i.e. the possibility of using the product without the risk of damaging, destroying or invalidating its use of the tool or surface to be treated.
-
Are there pathogens more resistant than mycobacteria?
Yes, the most difficult pathogens to eliminate are spores and prions, for to eliminate of which it is necessary to resort to the use of the autoclave for sterilization.
-
Are all disinfectants the same?
Disinfectants are not all the same, there are many different types based on their formulation and the active principle(s) contained therein.
-
Are all pathogens equally resistant to disinfectants?
No, pathogens have very different resistances depending on the disinfectant substance used to try to eliminate them. The least resistant pathogens range from the encapsulated viruses up to mycobacteria, which are instead the most resistant and require a high level of disinfection to eliminate them.
-
How do I choose the right disinfectant for my practice?
 Accessories
Accessories
-
Service Oil F1 (6)
-
Within what range of temperatures is W&H F1 Service Oil known to be stable?
W&H F1 Service Oil has longterm stability between –30 °C and +160 °C.
-
Which aerosol is used in the W&H F1 spray can and what properties does it have?
A mixture of propane and butane (a standard aerosol in all spray cans). It contains no CFCs so is environmentally-friendly.
-
How should I dispose of empty cans?
In accordance with the national regulations for aerosol cans. More information on disposal can be found on the safety datasheet.
-
Why is W&H oil better than other lubricants?
Extensive research and testing has resulted in a formula which best covers the extreme demands of dental handpieces. It consists of an allsynthetic extremely pure oil supplemented by a specially developed additive package and is designed to withstand the high temperatures and pressures encountered within the sterilization process.
-
Can W&H F1 Service Oil be sterilized?
Yes, W&H has researched its sterilizability and has found that W&H F1 Service Oil has no effect on the sterilization of the instruments.
-
Can I use any oil to lubricate my W&H instruments?
W&H recommends that W&H instruments are lubricated with W&H oil, as this has been designed and developed to enhanced the performance of your W&H instrument, and to protect the instrument during the high temperatures and pressures involved in the sterilization process.
-
Within what range of temperatures is W&H F1 Service Oil known to be stable?
-
Seal2 (1)
-
Should I pre-wrap and how long can I store my instruments after sterilization?
Under current UK decontamination guidance, with a type B vacuum cycle, instruments should be pre-wrapped and once sterilized can be stored for up to 12 months. Wrapping should take place shortly after washing and disinfection. Under current UK decontamination guidance, with a type N cycle, instruments should not be pre-wrapped. Once sterilized, instruments can be immediately aseptically wrapped and stored for up to 12 months. Instruments should always be dry before they are placed in the purpose-designed packaging.
-
Should I pre-wrap and how long can I store my instruments after sterilization?
-
Test kits (9)
-
Do I need to carry out periodic testing on my ThermoKlenz?
Yes, testing is an integral part of ensuring that a small sterilizer consistently performs to operating parameters set during the machines commissioning. Failure to carry out routine periodic tests and maintenance tasks could compromise safety and have legal and insurance-related implications for the registered Manager.
-
What periodic tests do I need to carry out on my ThermoKlenz?
Periodic testing is set out in the current national decontamination guildance document. In addition to a daily automatic control test and weekly safety inspection, other periodic tests should be performed by the operator or user and will normally consist of: - Protein Residue Test - Cleaning Efficacy Test (Residual Soil Detection Test) Additional information relating to these tests and how to carry out these tests can be found on the Video Guides section of this web site.
-
Does my ThermoKlenz need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
How do I clean my ThermoKlenz and what products should I use?
Information and videos relating to the cleaning of your ThermoKlenz and cleaning products can be found on the Video Tutorial section of this web site.
-
Who should I use to carry out validation and maintenance on my ThermoKlenz?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I de-scale my ThermoKlenz?
Information and videos relating to de-scaling your ThermoKlenz can be found on the Video Tutorial section of this web site.
-
Can I process all handpieces in my ThermoKlenz?
No, only instruments which have the thermal disinfection symbol can be processed, check with the manufacturer that a washer-disinfector can be used to clean the handpiece.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Do I need to carry out periodic testing on my ThermoKlenz?
-
ThermoKlenz consumables (10)
-
Do I need to carry out periodic testing on my ThermoKlenz?
Yes, testing is an integral part of ensuring that a small sterilizer consistently performs to operating parameters set during the machines commissioning. Failure to carry out routine periodic tests and maintenance tasks could compromise safety and have legal and insurance-related implications for the registered Manager.
-
What periodic tests do I need to carry out on my ThermoKlenz?
Periodic testing is set out in the current national decontamination guildance document. In addition to a daily automatic control test and weekly safety inspection, other periodic tests should be performed by the operator or user and will normally consist of: - Protein Residue Test - Cleaning Efficacy Test (Residual Soil Detection Test) Additional information relating to these tests and how to carry out these tests can be found on the Video Guides section of this web site.
-
Does my ThermoKlenz need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
How do I clean my ThermoKlenz and what products should I use?
Information and videos relating to the cleaning of your ThermoKlenz and cleaning products can be found on the Video Tutorial section of this web site.
-
Who should I use to carry out validation and maintenance on my ThermoKlenz?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I de-scale my ThermoKlenz?
Information and videos relating to de-scaling your ThermoKlenz can be found on the Video Tutorial section of this web site.
-
Can I process all handpieces in my ThermoKlenz?
No, only instruments which have the thermal disinfection symbol can be processed, check with the manufacturer that a washer-disinfector can be used to clean the handpiece.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Do I need to carry out periodic testing on my ThermoKlenz?
 Straight & Contra-angle Handpieces
Straight & Contra-angle Handpieces

-
Alegra (8)
-
Can the straight handpiece HE-43 E be used for surgical applications?
The new Alegra handpiece range have been tested and approved for the following applications: removal of decayed materials, cavities and crown cement, removal of fillings, finishing and polishing of tooth and restoration surfaces. Use in other areas of application is not recommended and therefore at user’s risk.
-
Can LED instruments with generator be sterilized?
Yes, W&H handpieces with LED and generator are both thermo washer disinfectable
 and sterilizable
and sterilizable  .
.
-
Can the current range of Alegra instruments be connected to motors with a light supply?
Yes, the straight and contra-angle handpieces in the new Alegra range can be used on any motor with an ISO connection.
-
What are the advantages of the Alegra instruments?
Independently generated LED light – the optic instruments in the Alegra range have LED light, which is supplied with energy from an integrated generator. The instruments are therefore independent of the light supply of the motor and the dental unit and offer the possibility of working with light regardless of which unit is used. The technology of the integrated generator means that there is only illumination when the instrument is running.
-
For what reason may the LED fail to light up?
Because the energy for the LED light in the new Alegra instruments is generated by an integrated generator, the brightness of the LED depends on the motor speed (at least 9,000rpm).
-
Is more heat generated by the light source when LED technology is employed?
No, because they are usually operated with coolant air. In addition, the LED contra-angle handpieces are equipped with an internal spray function, which guarantees additional cooling.
-
What is the difference between the Alegra contra-angle handpieces WE-56 T and WE-56?
Alegra contra-angle handpieces designated with the letter "T" are significantly lighter in weight. Alegra contra-angle handpieces that are not designated with the letter "T" have been approved for automated thermo washer disinfection.
-
Why does the WE-99 contra-angle handpiece have a transmission ratio of 1:4.5?
The optimum speed of fast-running burs can easily be achieved using the WE-99.
-
Can the straight handpiece HE-43 E be used for surgical applications?
 Surgical Devices
Surgical Devices

-
Implantmed (24)
-
Can handpieces from another manufacturer be used on a W&H surgical motor (i.e. Implantmed or Elcomed)?
For applications which do not require accurate torque (drilling, grinding,…), any handpiece with ISO 3964 connection can be used. Please be informed that handpieces from other manufacturers might not be able to handle the torque of your unit/motor and therefore might get damaged.
-
Can other brands of tubing be used?
This is not recommended. Due to different specifications (material, dimensions etc) it could cause problems like leakage, insufficient cooling or even damage the unit. For details of the correct tubing to use with your unit see "correlation of tubing" or user manual.
-
How to insert irrigation tube?
Please find a detailed description in the user manual at chapter "Starting operation - General".
-
How can the unit / motor be cleaned / sterilized?
Please find a detailed description in the user manual chapter on "Hygiene and Maintenance".
-
Is it possible to adjust the motor speed within the program to insert an implant?
No, the speed in program P4 is 15 rpm when in forward operation and 30 rpm in reverse operation. In program P5 (thread cutter function) the speed is 20 rpm in both forward and reverse operation and only the torque can be set.
-
Why is the light of my "LED G" handpiece not working when placing the implant?
The LED light is generated by the handpiece; it needs at least 300 rpm to produce enough power for the LED to light up.
-
Can my Implantmed/Elcomed motor be processed in a thermo washer disinfector?
Yes, the current generation of Implantmed and Elcomed motors can be, providing the thermo washer disinfector has a drying cycle and the motor bears the sign that it is "Thermo washer disinfectable".

-
Can my Implantmed/Elcomed motor be processed in a vacuum sterilizer with the protection cap in place.
No. The current generation of motors have to be sterilized without the cap.
-
Does my W&H unit need to be serviced.
Regular servicing of function and safety including the accessories is necessary and we recommend it is carried out at least once a year, unless shorter intervals are prescribed by law or local guidelines. Detailed information can be found in the user manual at chapter "Servicing".
-
What types of tubing can I use on my device?
For proper tubing see "correlation of tubing" or user manual.
-
Does the motor have to be lubricated?
No. The bearings in the motor are lubed for life; any kind of lubrication would shorten the lifespan of the motor.
-
What’s the difference between torque and ISQ?
Torque is a one time, static measurement at the time of placement of the implant and cannot be repeated later on in a non-invasive way. Osstell ISQ measures the lateral stability of the implant and the measurements can be repeated in a non-invasive, dynamic way to monitor the development of osseointegration.
-
Is every new Implantmed compatible with the W&H Osstell ISQ module?
Yes. All control units SI-1023 can be upgraded with the W&H Osstell ISQ module
-
Are previous Implantmed motors compatible with the new Implantmed or vice versa?
No. The new motor has a new technological basis and is the shortest available
-
What does the LC in EM-19 LC stand for, is there an LED in the motor?
The LC stands for Light Contacts. This means that we have integrated an electrical connection into our new motors. The Light Contacts deliver power to our new contra-angles to offer our customers the best light at any rotation speed. For oral surgery handpieces from W&H, the LED remains in the head of the contra-angles
-
Can current contra-angles be attached to new motors?
Yes. All W&H surgical contra-angles and handpieces can be attached to the new motor EM-19.
-
Can the new contra-angles be attached to old motors?
No. In order to deliver the best balance we shortened the inner coupling length. Therefore surgical contra-angles with only the 'L' suffix cannot be attached to old motors
-
Are old cable foot controls compatible with the new unit or vice versa?
No. The new cable foot control S-N2 is based on the industrial CAN standard, therefore connecting the old foot control S-N1 is not possible.
-
Can previous Implantmed units be retrofitted with the wireless foot control
It depends but basically yes. The following units can be retrofitted
Implantmed SI-923 REF16929000
Implantmed SI-915 REF16929001
Elcomed SA-310 REF15933100/15933101/15933102/15933103
-
Is it possible to use the Implantmed Classic in combination with the wireless foot controller?
No, the SPI dongle does not fit.
-
Piezomed module: Are the handpieces of the modules compatible with the SA-320?
No, not compatible.
-
Piezomed module: Does the module also work with OEM devices?
Yes, but different software updates are necessary.
-
Piezomed module: Can devices be retrofitted on the market?
Yes, all SI-10XX devices.
-
Can our customers dis-assemble the surgical contra-angles
Yes. At W&H the letter "S" indicates that the surgical contra-angle can be dis-assembled for sterilization. The letter "I" indicates that the surgical contra-angle cannot be dis-assembled (e.g. WS-75 L vs. WI-75)
-
Can handpieces from another manufacturer be used on a W&H surgical motor (i.e. Implantmed or Elcomed)?
-
Piezomed (11)
-
How are instruments prepared for cleaning?
a. Preparation in the ultrasonic bath:
- Place the instruments in the instrument tray and lower into the ultrasonic bath.
- Use cleaning agents and disinfectants suitable for hand instruments (probes, mirrors, forceps, etc.).
DO NOT USE a bur bath, as this is very aggressive.
- After cleaning in the ultrasonic bath, rinse adequately with water to remove any remnants of the cleaning agent and disinfectant from the coolant channels.
- Blow dry with compressed air after rinsing
- Replace dried instruments in the instrument tray and package for sterilization
b. Mechanical preparation in the cleaning unit and disinfector
- Use the cleaning adapter for cleaning units and disinfectors (see Instructions for Use – Accessories)
- After preparation in the cleaning unit and disinfector, check that the coolant channels are dry and, if necessary, dry again with compressed air. -
Can third-party instruments be used on the Piezomed?
No
a. Different thread
b. The Piezomed instrument detection cannot detect third-party instruments
-
How long is the useful service life of the LED?
The user must replace the LED socket as soon as the protective coating on the LED discolours and the luminous power is impaired as a result. The length of time this takes can differ depending on the type of sterilizer or sterilization process.
The instrument detection?
The instrument detection is located in the LED socket and is automatically replaced at the same time as the LED socket. -
Can Piezomed instruments also be used on third-party devices?
No
a. Different thread
b. Third-party device cannot find the correct resonance point – instrument fails to oscillate -
How long do the instruments last and when should they be replaced?
The useful service life of the instruments is determined by the length of time they are used reprocessing. Sterilization cycles have only marginal influence.
a. Instruments need to be replaced
- If there is a loss of power while working
- Where there is visible damage on the working part or the shank of the instrument.
b. Saws must be replaced if
- Teeth are broken off
- Teeth are worn
c. Diamond-coated instruments must be replaced
- As soon as the diamond coating is worn
-
How long is the useful service life of the instrument detection?
The instrument detection is located in the LED socket and is automatically replaced at the same time as the LED socket.
-
Piezomed module: Are the handpieces of the modules compatible with the SA-320?
No, not compatible.
-
Piezomed module: Does the module also work with OEM devices?
Yes, but different software updates are necessary.
-
Piezomed module: Can devices be retrofitted on the market?
Yes, all SI-10XX devices.
-
Piezomed module: Compatibility of the handpieces with Piezomed Plus module
and Piezomed Classic module.
Handpieces Piezomed Plus module Piezomed Classic module SA-40 yes yes SA-40 L (light version) yes no SA-320 no no -
Piezomed module: What are the main differences between the Piezomed Plus module
and Piezomed Classic module?
Features Piezomed Plus module Piezomed Classic module Power 24 watts 18 watts Light LED no light Tip detection yes no Colour black white Cable length 1.8 m and 3.5 m 1.8 m
-
How are instruments prepared for cleaning?
-
Elcomed (10)
-
Can handpieces from another manufacturer be used on a W&H surgical motor (i.e. Implantmed or Elcomed)?
For applications which do not require accurate torque (drilling, grinding,…), any handpiece with ISO 3964 connection can be used. Please be informed that handpieces from other manufacturers might not be able to handle the torque of your unit/motor and therefore might get damaged.
-
Can other brands of tubing be used?
This is not recommended. Due to different specifications (material, dimensions etc) it could cause problems like leakage, insufficient cooling or even damage the unit. For details of the correct tubing to use with your unit see "correlation of tubing" or user manual.
-
How to insert irrigation tube?
Please find a detailed description in the user manual at chapter "Starting operation - General".
-
How can the unit / motor be cleaned / sterilized?
Please find a detailed description in the user manual chapter on "Hygiene and Maintenance".
-
Why is the light of my "LED G" handpiece not working when placing the implant?
The LED light is generated by the handpiece; it needs at least 300 rpm to produce enough power for the LED to light up.
-
Can my Implantmed/Elcomed motor be processed in a thermo washer disinfector?
Yes, the current generation of Implantmed and Elcomed motors can be, providing the thermo washer disinfector has a drying cycle and the motor bears the sign that it is "Thermo washer disinfectable".

-
Can my Implantmed/Elcomed motor be processed in a vacuum sterilizer with the protection cap in place.
No. The current generation of motors have to be sterilized without the cap.
-
Does my W&H unit need to be serviced.
Regular servicing of function and safety including the accessories is necessary and we recommend it is carried out at least once a year, unless shorter intervals are prescribed by law or local guidelines. Detailed information can be found in the user manual at chapter "Servicing".
-
What types of tubing can I use on my device?
For proper tubing see "correlation of tubing" or user manual.
-
Does the motor have to be lubricated?
No. The bearings in the motor are lubed for life; any kind of lubrication would shorten the lifespan of the motor.
-
Can handpieces from another manufacturer be used on a W&H surgical motor (i.e. Implantmed or Elcomed)?
 Straight & Contra-angle Handpieces
Straight & Contra-angle Handpieces

-
Contra-angles (9)
-
What material is used for the manufacture of W&H surgical instruments?
Internal and external parts are made of high quality stainless steel (including the contra-angle handpiece head).
-
Can LED instruments with generator be sterilized?
Yes, W&H handpieces with LED and generator are both thermo washer disinfectable
 and sterilizable
and sterilizable  .
.
-
How to dismantle the handpiece
See detailed description in user manual or Video.
-
Why is the light of my "LED G" handpiece not working when placing the implant?
The LED light is generated by the handpiece; it needs at least 300 rpm to produce enough power for the LED to light up.
-
Is the black or green spray clip of my surgical handpiece sterilizable?
Yes. In order to extend the live span of the clip, W&H recommends to remove the clip from the handpiece and sterilize it separately (handpiece and clip in one pouch but separated).
-
What is the difference between WI-75 and WS-75?
These surgical contra-angle handpieces have similar performance. However, the WS-75 can be dismantled which means it can be cleaned more thoroughly.
-
Can current contra-angles be attached to new motors?
Yes. All W&H surgical contra-angles and handpieces can be attached to the new motor EM-19.
-
Can the new contra-angles be attached to old motors?
No. In order to deliver the best balance we shortened the inner coupling length. Therefore surgical contra-angles with only the 'L' suffix cannot be attached to old motors
-
Can our customers dis-assemble the surgical contra-angles
Yes. At W&H the letter "S" indicates that the surgical contra-angle can be dis-assembled for sterilization. The letter "I" indicates that the surgical contra-angle cannot be dis-assembled (e.g. WS-75 L vs. WI-75)
-
What material is used for the manufacture of W&H surgical instruments?
-
Handpieces (8)
-
What material is used for the manufacture of W&H surgical instruments?
Internal and external parts are made of high quality stainless steel (including the contra-angle handpiece head).
-
Can LED instruments with generator be sterilized?
Yes, W&H handpieces with LED and generator are both thermo washer disinfectable
 and sterilizable
and sterilizable  .
.
-
What is the minimum shaft length of the bur for the S-12?
The shaft of the bur should be at least 53mm long.
-
How to dismantle the handpiece
See detailed description in user manual or Video.
-
Can current contra-angles be attached to new motors?
Yes. All W&H surgical contra-angles and handpieces can be attached to the new motor EM-19.
-
Can the new contra-angles be attached to old motors?
No. In order to deliver the best balance we shortened the inner coupling length. Therefore surgical contra-angles with only the 'L' suffix cannot be attached to old motors
-
Can our customers dis-assemble the surgical contra-angles
Yes. At W&H the letter "S" indicates that the surgical contra-angle can be dis-assembled for sterilization. The letter "I" indicates that the surgical contra-angle cannot be dis-assembled (e.g. WS-75 L vs. WI-75)
-
What is the minimum shaft length of the bur for the S-11 Surgical Handpiece?
The shaft of the bur should be at least 44mm long (ISO-shaft 104).
-
What material is used for the manufacture of W&H surgical instruments?
 Turbines
Turbines

-
Synea (10)
-
Are LED turbines from the current Synea series compatible with previous models of Roto Quick coupling (e.g. 924)?
Yes, the new generation of turbines can be operated with both current and previous models of coupling.
-
Which burs can be used with W&H turbines?
Mini head from 16 mm – 21 mm (i.e. all standard bur lengths) and midi/standard heads 19 mm – 25 mm burs (e.g. burs for special applications) with a maximum cutting diameter of 2 mm. Note that the specifications of the bur manufacturer must be observed. When using longer rotary instruments the user must ensure, by selection of appropriate operating conditions, that there is no danger to the user, patient or third parties.
-
How does the new W&H Click & Pull system work? How do I remove a turbine?
Use your thumb and index finger to retract the coupling's retention sleeve. The turbine can now be removed from the coupling without any problems. Pulling forcefully on the dental turbine can cause damage. If excessive force is required to remove the turbine, verify that the retention sleeve on the coupling can be moved without any problems.
-
Why do I need a 6-hole connection to operate a standard LED turbine?
A 6-hole connection is necessary to supply the turbine with electricity. Other connections do not provide a power supply. The only turbines that do not require a 6-hole connection are the self-generating Alegra LED G turbines which can also be used on non-optic connections.
-
What is the maximum length of rotary instruments that can be used with the handpiece?
25 mm for the standard turbine (XX-98 models) and 21 mm for turbines with small head (XX-97 models). When using longer rotary instruments the user must ensure by correct selection of the operating conditions, that there is no danger to the user, patient or third parties.
-
I've just bought a new W&H turbine? Should I call a service engineer for my unit to install it?
Whilst many users will use their handpieces as soon as they have removed them from the packaging and processed them through a decontamination cycle, it is advisable to instruct an engineer to ensure that the air/water pressure and if applicable the voltage for the turbine are set correctly, to prevent the risk of instrument failure or damage.
-
At the moment I have a W&H turbine without light. How can I have a light in my turbine?
If there is electricity at the turbine hose, you need a proper coupling and a turbine with light (example: RQ-24 and TG-98 L). If no electricity is available, the simplest way is to buy W&H Alegra LED G turbine which has a generator and LED (light-emitting diode) built in. It doesn't require any other investment in the dental unit.
-
My turbine doesn't have enough power/speed?
Please check drive air pressure on your unit and maintenance of the instruments (Synea with RQ connection = 3 +/- 0.3 bar, with MULTIflex® connection 2.5 - 4 bar). For all Alegra turbines the pressure needs to be set between 2.5 - 2.8 bar.
-
Can my W&H turbine be sterilized?
Yes, providing that your W&H turbine carries the relevant sterilization symbol.

-
Can my W&H turbine be processed via a thermo washer disinfector?
Yes, providing that your W&H turbine carries the relevant thermo washer disinfection symbol
 ; also the thermo washer disinfector must have a drying cycle.
; also the thermo washer disinfector must have a drying cycle.
-
Are LED turbines from the current Synea series compatible with previous models of Roto Quick coupling (e.g. 924)?
-
Alegra (10)
-
Which burs can be used with W&H turbines?
Mini head from 16 mm – 21 mm (i.e. all standard bur lengths) and midi/standard heads 19 mm – 25 mm burs (e.g. burs for special applications) with a maximum cutting diameter of 2 mm. Note that the specifications of the bur manufacturer must be observed. When using longer rotary instruments the user must ensure, by selection of appropriate operating conditions, that there is no danger to the user, patient or third parties.
-
How does the new W&H Click & Pull system work? How do I remove a turbine?
Use your thumb and index finger to retract the coupling's retention sleeve. The turbine can now be removed from the coupling without any problems. Pulling forcefully on the dental turbine can cause damage. If excessive force is required to remove the turbine, verify that the retention sleeve on the coupling can be moved without any problems.
-
What is the maximum length of rotary instruments that can be used with the handpiece?
25 mm for the standard turbine (XX-98 models) and 21 mm for turbines with small head (XX-97 models). When using longer rotary instruments the user must ensure by correct selection of the operating conditions, that there is no danger to the user, patient or third parties.
-
I've just bought a new W&H turbine? Should I call a service engineer for my unit to install it?
Whilst many users will use their handpieces as soon as they have removed them from the packaging and processed them through a decontamination cycle, it is advisable to instruct an engineer to ensure that the air/water pressure and if applicable the voltage for the turbine are set correctly, to prevent the risk of instrument failure or damage.
-
At the moment I have a W&H turbine without light. How can I have a light in my turbine?
If there is electricity at the turbine hose, you need a proper coupling and a turbine with light (example: RQ-24 and TG-98 L). If no electricity is available, the simplest way is to buy W&H Alegra LED G turbine which has a generator and LED (light-emitting diode) built in. It doesn't require any other investment in the dental unit.
-
My turbine doesn't have enough power/speed?
Please check drive air pressure on your unit and maintenance of the instruments (Synea with RQ connection = 3 +/- 0.3 bar, with MULTIflex® connection 2.5 - 4 bar). For all Alegra turbines the pressure needs to be set between 2.5 - 2.8 bar.
-
Can my W&H turbine be sterilized?
Yes, providing that your W&H turbine carries the relevant sterilization symbol.

-
Can my W&H turbine be processed via a thermo washer disinfector?
Yes, providing that your W&H turbine carries the relevant thermo washer disinfection symbol
 ; also the thermo washer disinfector must have a drying cycle.
; also the thermo washer disinfector must have a drying cycle.
-
Does the Alegra turbine handpiece also product light when used on optic dental units?
Alegra LQ LED turbine handpieces are exclusively intended for use with Roto Quick couplings RQ-54 / RQ-53. Alegra LQ LED turbine handpieces cannot be coupled onto Roto Quick couplings RQ-24 / RQ-34.
-
I have a new Alegra LQ LED turbine handpiece, which is supplied with power by a Roto Quick coupling with integrated generator. What should I pay attention to when servicing the Roto Quick coupling with generator?
The generator in the coupling is equipped with ball bearings, which must be lubricated once a month. This sufficiently removes any soiling from the generator.
-
Which burs can be used with W&H turbines?
 Reprocessing Devices
Reprocessing Devices

-
Assistina TWIN (6)
-
Why is there no rotational lubrication option in the Assistina TWIN?
The oil is atomized in the Assistina TWIN before it enters the instrument. This ensures full coverage of the gear parts but eliminates the need for technically complex and time-consuming rotational lubrication. There is no negative impact on the quality of maintenance with the high level results corresponding to those achieved using the Assistina 301 Plus.
-
What instrument adaptors are available for the Assistina TWIN?
All adaptors that are suitable for the Assistina 3x2/3x3 can also be used in the Assistina TWIN. There is only one exception: the RM/ISO adaptor for the Assistina 3x2/3x3 is not compatible with the Assistina TWIN. Quick Connect adaptors ISO and RM can be used for the Assistina TWIN instead. An overview of all adaptors is available on the product website.
-
What does HEPA mean, and what is filtered out of the air?
HEPA is short for 'High Efficiency Particular Air', and the filter is classified as HEPA class E11. This means that the filter retains all particles larger than 1 µm, which includes, among other things, a wide range of bacteria and viruses, suspended particles such as dust and respirable aerosols.
-
How does the user know when the TWIN Care Set needs to be replaced?
The intelligent process monitoring system documents the number of maintenance cycles that have been performed, thereby automatically calculating the number of instruments that can be serviced with the remaining quantity of fluid. The user receives an alert 300 cycles before the cartridge needs replacing (the oil and cleaner LEDs on the top of the unit turn yellow in colour).
-
How often does the TWIN Care Set need to be replaced?
The consumption volumes for each instrument are 0.07 ml of oil and 0.07 ml of cleaning solution. This gives a maximum capacity of 2857 cycles. In reality, the value is around 2800 to 2850 cycles, as initial filling is also required and the fill quantity of the cartridges is subject to certain tolerances that are regulated by the applicable standards.
-
Why does the Assistina TWIN have a HEPA filter?
During the Assistina TWIN's handpiece maintenance process the rotating parts are lubricated with oil and the spray channels rinsed with cleaning solution. These liquids are introduced to the handpieces using compressed air, which produces aerosols that may contain potentially contaminated body fluids (e.g. blood and saliva). In order to ensure that the apparatus poses no risk to the user, the patient or third parties, aerosols must be prevented from leaking out of the apparatus. We make sure of this by using active suction and the HEPA filter. This HEPA filter needs to be changed regularly, which is why the lifespan of the filter is matched exactly to the capacity of the two cartridges. The HEPA filter therefore needs changing every time that the refill cartridge set is replaced.
-
Why is there no rotational lubrication option in the Assistina TWIN?
-
Assistina 301 plus (4)
-
Correct use of Assistina 301plus.
Please find a detailed description about first startup, regular use and maintenance in the user manual. Furthermore you can find video tutorials about Assistina 301plus.
-
How can I check if my Assistina 301 or 301plus is working properly?
Check/replace o-rings on motor coupling/adaptor. Make sure that the "Start" button is held down for at least 2 sec. when starting a cycle. When pressing the button, the green ball at the function indicator on the right (cleaning solution) should show up. After keeping the button depressed for 2 sec., the green ball on the left should show up. A detailed function check can be found at the "weekly checklist" and the user manual at chapter "Test" and "Function Check".
-
How much air pressure does the Assistina 301 plus require in order to operate properly?
The pressure should be between 4 - 10 bar (58 to 145 psi).
-
How often should the filter in the supply air be changed (Assistina 301 plus)?
Annually or if the filter is full of debris (visual check - filter should be white). Please see video about changing the filter).
-
Correct use of Assistina 301plus.
-
Assistina ONE (8)
-
How does the user know when the ONE Care Set needs to be replaced?
The ONE Care Set must be replaced when the cartridges are empty. The levels can be checked thanks to the device's fill level indicators, located on the right and left sides.
-
How do you replace the ONE Care Set?
The two cartridges and the filter can be changed easily without any tools. The fluid is supplied via tapping needles in the bottom of the cartridge spaces that penetrate the cartridge membrane, thereby drawing the fluid out of the cartridge. The two cartridges have different shapes to ensure that the two fluid types cannot be mixed up. In addition, it is only possible to install the HEPA filter incorrectly by using force, which is not necessary.
-
Why there is no rotational lubrication option in the Assistina One?
The oil is atomized in the Assistina One before it enters the instrument. This ensures full coverage of the gear parts, eliminating the need for technically complex and time-consuming rotational lubrication. However, there is no negative impact on the maintenance quality.
-
What does HEPA mean, and what is filtered out of the air?
HEPA is short for 'High Efficiency Particular Air', and the filter is classified as HEPA class E11. This means that the filter retains all particles larger than 1 µm, which includes, among other things, a wide range of bacteria and viruses, suspended particles such as dust and respirable aerosols.
-
What is the difference between oil nebulization and rotational lubrication?
Final result is the same, but in a shorter time. In the nebulization system, oil is nebulized in the adaptor before it is blown into the handpiece. An oil mist coats all gear parts.
-
What instrument adaptors are available for the Assistina One?
All adaptors that are suitable for the Assistina 3x2/3x3 can also be used in the Assistina One. There is only one exception: the RM/ISO adaptor for the Assistina 3x2/3x3 is not compatible with the Assistina One. Quick Connect adaptors ISO and RM can be used for the Assistina One instead. An overview of all adaptors is available in the product website.
-
Why does the Assistina One have a HEPA filter?
During the maintenance process in the Assistina One, the rotating parts are lubricated with oil, and the spray channels rinsed with cleaning solution. These liquids are introduced to the handpieces using compressed air, which produces aerosols that may contain potentially contaminated body fluids (e.g. blood and saliva). In order to ensure that the apparatus poses no risk to the user, the patient or third parties, the legislation stipulates that aerosols must be prevented from leaking out of the apparatus. We make sure of this by using active suction and the HEPA filter. This HEPA filter must of course be changed regularly, which is why the lifespan of the filter is matched exactly to the capacity of the two cartridges. The HEPA filter therefore reaches the end of its service life once one set of cartridges has been used.
-
How often does the ONE Care Set needs to be replaced?
The consumption volumes for each instrument are 0.07 ml of oil and 0.07 ml of cleaning solution. This gives a maximum capacity of 2857 cycles. The value is around 2800 to 2850 cycles, as initial filling is also required and the fill quantity of the cartridges is subject to certain tolerances that are regulated by the applicable standards.
-
How does the user know when the ONE Care Set needs to be replaced?
-
ThermoKlenz (9)
-
Do I need to carry out periodic testing on my ThermoKlenz?
Yes, testing is an integral part of ensuring that a small sterilizer consistently performs to operating parameters set during the machines commissioning. Failure to carry out routine periodic tests and maintenance tasks could compromise safety and have legal and insurance-related implications for the registered Manager.
-
What periodic tests do I need to carry out on my ThermoKlenz?
Periodic testing is set out in the current national decontamination guildance document. In addition to a daily automatic control test and weekly safety inspection, other periodic tests should be performed by the operator or user and will normally consist of: - Protein Residue Test - Cleaning Efficacy Test (Residual Soil Detection Test) Additional information relating to these tests and how to carry out these tests can be found on the Video Guides section of this web site.
-
Does my ThermoKlenz need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my ThermoKlenz?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I de-scale my ThermoKlenz?
Information and videos relating to de-scaling your ThermoKlenz can be found on the Video Tutorial section of this web site.
-
Can I process all handpieces in my ThermoKlenz?
No, only instruments which have the thermal disinfection symbol can be processed, check with the manufacturer that a washer-disinfector can be used to clean the handpiece.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Do I need to carry out periodic testing on my ThermoKlenz?
 Cordless Devices
Cordless Devices

-
Proxeo Twist Cordless (8)
-
How long is the battery life of the Proxeo Twist Cordless?
The rechargeable battery normally lasts for around one working day (8–12 patients each with a polishing duration of 4–6 minutes [actual time the foot control is ‘on’]).
-
Is it the same rechargeable battery as a smartphone and what is the rechargeable battery’s capacity in comparison to the lifespan of the medical device? With a smartphone, I have to charge more often the older it gets.
The Proxeo Twist Cordless has a Li-ion rechargeable battery (like many smartphones). That said, you can’t compare the Proxeo Twist Cordless with a smartphone in this context. Smartphones have apps and updates installed on them which require more processing capacity and are also updated in the background which shortens the battery life and means it needs to be recharged more often, even though it only loses a small amount of capacity.
-
Are there any recommendations for charging the Proxeo Twist Cordless’ rechargeable battery?
Yes, we recommend charging the battery regularly through the day (e.g. during the lunch break), in order to achieve optimal performance.
-
How long does the foot control’s rechargeable battery last?
The rechargeable battery lasts approx. two months based on average use (5 working days with 8–12 patients/day, used for 4–6 minutes per patient).
-
Is it possible to thermally disinfect and sterilize the Proxeo Twist Cordless?
The Proxeo Twist Cordless can be fully disinfected by wiping down. The handpiece sleeve can be sterilized and thermally disinfected. The foot control can also be disinfected by wiping down. You will find further information on this in the Instructions for use 50928.
-
Which prophy angle cups can be used with the Proxeo Twist Cordless?
The Proxeo Twist prophy angle cups from W&H have some outstanding benefits. You can, however, use all conventional prophy angle cups with the Doriot system.
-
How can I connect the Proxeo Twist Cordless with the foot control?
You will find further information on this in the Instructions for use 50928.
-
Should I always call my service department before sending devices/instruments for repair?
It is always best to contact your service department first and talk to a technician as they may be able to resolve the issue or can let you know which items needs to be sent for repair.
-
How long is the battery life of the Proxeo Twist Cordless?
 Straight & Contra-angle Handpieces
Straight & Contra-angle Handpieces

-
Proxeo TWIST (5)
-
Can my W&H handpiece be processed via a thermo washer disinfector and sterilizer.
Yes, providing that your W&H instrument carries the relevant thermo washer disinfection and sterilization symbols and the thermo washer disinfector has a drying cycle.

-
Can my W&H handpiece be sterilized?
Yes, providing that your W&H handpiece carries the relevant sterilization symbol.

-
Should I call the service station before sending equipment for repair?
If you are not sure what the cause of the problem is, it is better to call the service station and talk to a technician. Together with the technician you can determine what needs to be sent in, or sometimes the problem can even be resolved over the phone.
-
Can I use any Prophy cup that is available on the market for the WP-66 W?
In order to benefit from the advantages of the LatchShort system and to support the longevity of the contra-angle handpiece (WP-66 W), we recommend using W&H Prophy cups. However, it is possible for Prophy cups from standard latch systems (with a shaft of 2.35 mm) to be used.
-
Why should I use a prophylaxis contra-angle handpiece (WP-66 W)?
Generally, prophylaxis contra-angle handpieces feature a seal on the head that prevents the ingress of saliva and paste. This extends the lifespan of the contra-angle handpiece.
The most effective removal of discolouration that at the same time protects the enamel and dentine as much as possible, depends on the polishing speed, i.e. revolutions per minute, among other things. With a speed ratio of 4:1 (four revolutions of the motor mean one revolution of the cup), the revolution speed of the Prophy cup is reduced. This means during application, there is no risk of paste splattering.
-
Can my W&H handpiece be processed via a thermo washer disinfector and sterilizer.
 Water Treatment Devices
Water Treatment Devices

-
Multidem (2)
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
Can the empty Multidem C27 cartridge be returned or must this be disposed of yourself (household waste)?
The products must be disposed in accordance with current local, regional and country regulations; see the disposal considerations (Section 11) contained in the Product Safety Information Sheet.
-
What water quality should I use with my Lisa/Lina?
-
Osmo (1)
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
What water quality should I use with my Lisa/Lina?
 Piezo Scaler
Piezo Scaler

-
Proxeo Ultra (14)
-
Can I disinfect the tank by wiping it down?
Yes
-
Can I disinfect the tank by putting it in the thermo washer?
No
-
Can I treat patients with cardiac pacemakers with W&H Piezo scalers?
Yes, the compatibility of these devices has been assessed in application.
-
How long does the foot control’s rechargeable battery last?
The rechargeable battery lasts approx. two months based on average use (5 working days with 8–12 patients/day, used for 4–6 minutes per patient).
-
Should I call the service department before I send devices/instruments for repair?
It is always best to contact your service department first and talk to a technician as they may be able to resolve the issue over the phone or can let you know which items needs to be sent for repair.
-
Are W&H tips (e.g. 1U) compatible with the new W&H high-speed mounting system?
No, they are not compatible because they have different threads.
-
How do I know which tip goes with which handpiece?
There are symbols on the tip, the tip changer and the handpiece identifying which system they belong to.
-
Are the new handpieces compatible with the PA-123/PA-115?
No, they are not compatible.
-
Are the new handpieces (PB-5 L, PB-5 L S, PB-5 L Q) compatible with the earlier generation of Piezo scalers?
No, they are not compatible.
-
Can the handpiece be put through the sterilizer?
Yes, as long as your handpiece or contra-angle handpiece carries the mark “sterilizable up to the stated temperature”.
-
Is the handpiece thermo washer disinfectable?
Yes, as long as your handpiece or contra-angle handpiece carries the mark “thermo washer disinfectable”.
-
Can I put the handpiece in a universal hygiene devices (ie DAC-Universal)?
Yes
-
How can I recharge the wireless foot control (PB-530)?
The wireless foot control can be recharged by plugging the power pack directly into the device.
-
How can I connect the wireless foot control with the Proxeo ULTRA PB-530?
Set the power regulator to “OFF”. Connect the control unit and the foot control using the cable. Press and hold the function button for 5 seconds.
-
Can I disinfect the tank by wiping it down?
 Sterilizers
Sterilizers

-
MS Sterilizer (3)
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How often should I de-scale my Thermoklenz?
Water hardness can differ depending of the region in which the unit is located. Carrying out a water hardness test will enable you to gauge how hard the incoming water is. The frequency of de-scaling cycles will depend on the water hardness and the number of cycles run per week. W&H recommends that at least one de-scaling cycle be run each week. More frequent de-scaling cycles should be run if the washer disinfector has a heavier workload or if the water is hard. Additional information relating to these tests and how to carry out these tests can be found on the Video Tutorial section of this web site.
-
What is validation?
-
Lisa Mini (13)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Does my Lisa/Lina need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my Lisa/Lina?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
Should I pre-wrap and how long can I store my instruments after sterilization?
Under current UK decontamination guidance, with a type B vacuum cycle, instruments should be pre-wrapped and once sterilized can be stored for up to 12 months. Wrapping should take place shortly after washing and disinfection. Under current UK decontamination guidance, with a type N cycle, instruments should not be pre-wrapped. Once sterilized, instruments can be immediately aseptically wrapped and stored for up to 12 months. Instruments should always be dry before they are placed in the purpose-designed packaging.
-
What is the minimum shaft length of the bur for the S-11 Surgical Handpiece?
The shaft of the bur should be at least 44mm long (ISO-shaft 104).
-
What is the maximum noise level of the Lisa Mini sterilizer?
The max. noise level of Lisa Mini is 64 dB.
-
How do I clean the water tank and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu.
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu.
-
Is Lisa Mini requiring compressed air connection?
Lisa Mini requires compressed air connection to perform sterilization cycle. The compressed air specifications are available in the Instruction for Use, chapter “Technical data”.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
-
Lara (24)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Does my Lisa/Lina need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my Lisa/Lina?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
How do I clean the water tanks and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
Should I pre-wrap and how long can I store my instruments after sterilization?
Under current UK decontamination guidance, with a type B vacuum cycle, instruments should be pre-wrapped and once sterilized can be stored for up to 12 months. Wrapping should take place shortly after washing and disinfection. Under current UK decontamination guidance, with a type N cycle, instruments should not be pre-wrapped. Once sterilized, instruments can be immediately aseptically wrapped and stored for up to 12 months. Instruments should always be dry before they are placed in the purpose-designed packaging.
-
Lisa 17/22
No cycles are stored in the cycle history menuPOSSIBLE CAUSE
An electronic board was replaced by service.
SOLUTION
None. The memory of the old board cannot be restored. Save periodically the history on the USB pen drive. -
Lisa 17/22
The sterilizer enters into “Sleep mode” immediately after opening the chamber door.POSSIBLE CAUSE
The chamber door has not been opened after the previous cycle had finished and the “Sleep mode delay” has expired
SOLUTION
Press the SLEEP MODE button to exit”. -
Lisa 17/22
The sterilizer remains switched OFF.POSSIBLE CAUSE
The main switch or network circuit breaker is OFF
SOLUTION
Activate the main switch or network circuit breaker (ON).
POSSIBLE CAUSE
No voltage at the socket
SOLUTION
Check the electric circuit.
POSSIBLE CAUSE
The power cord is not connected properly
SOLUTION
Check and connect the power cord properly. -
Lisa 17/22
Water is leaking at the front of the sterilizerPOSSIBLE CAUSE
Leaks through the chamber door seal
SOLUTION
Clean or replace the door seal. Clean the chamber face side.
POSSIBLE CAUSE
Internal leak.
SOLUTIONS
Call technical service. -
Lisa 17/22
At the end of the cycle, there is residual water in the chamberPOSSIBLE CAUSE
Sterilizer not properly levelled
SOLUTION
Properly level the surface the sterilizer is placed on.
POSSIBLE CAUSE
Overloaded chamber
SOLUTION
Comply with the maximum load weight limits for each type of load. Always use the chamber rack for trays and cassettes.
POSSIBLE CAUSE
Chamber filter clogged
SOLUTION
Remove and clean the chamber filter.
POSSIBLE CAUSE
Chamber filter cap mispositioned
SOLUTION
Mount the chamber filter cap properly (see chapter MAINTENANCE)
POSSIBLE CAUSE
Load incorrectly placed
SOLUTION
Follow the recommendations as listed in ANNEX 2. -
Lisa 17/22
Corrosion or spots on instrumentsPOSSIBLE CAUSE
Tap water on instruments when placed in the sterilizer
SOLUTION
Ensure that instruments are dry before they are placed in the sterilizer.
POSSIBLE CAUSE
Use of water of poor quality or water containing chemical substances
SOLUTION
Drain both water tanks. Use water of good quality (see ANNEX 7).
POSSIBLE CAUSE
Organic or chemical residues on the instruments
SOLUTION
Clean, rinse and dry instruments before placing them in the sterilizer (see ANNEX 2).
POSSIBLE CAUSE
Chamber, trays, tray rack dirty
SOLUTION
Clean the chamber and wash the chamber furniture
POSSIBLE CAUSE
Contact between instruments of different materials
SOLUTION
Ensure that instruments of different materials do not touch (aluminum, carbon or stainless steel, etc.); place them on different trays or cassettes or pouch them (refer to ANNEX 2).
POSSIBLE CAUSE
Scale deposits on the chamber
SOLUTION
Clean the chamber and use water of good quality (refer to ANNEX 7). -
Lisa 17/22
When starting a cycle, the chamber door locks but re-opens immediately. The “Open the door” message appears.POSSIBLE CAUSE
Door seal not properly placed; seal sticking out
SOLUTION
Ensure that the door seal is correctly inserted around the whole circumference.
POSSIBLE CAUSE
Door jammed by external objects or by the load itself
SOLUTION
Remove any objects interfering with the chamber door. Check that the door does not push against the load or the chamber furniture. -
Lisa 17/22
Warning about programmed maintenance.POSSIBLE CAUSE
A component shall be replaced for the programmed maintenance of the sterilizer.
SOLUTION
Call service to order the requested component (door seal, dust filter, bacteriological filter...). See “Maintenance” chapter.. -
Lisa 17/22
When the sterilizer is connected to an automated water supply system: there is no clean water in the tank, but the automatic water filling does not fill the water.POSSIBLE CAUSE
Water fill system not connected
SOLUTION
Connect the water fill system to the sterilizer (see ANNEX 7 for water quality requirements).
POSSIBLE CAUSE
When the water fill system attempted to fill the tank, water was temporarily unavailable
SOLUTION
Since water tank filling is attempted only once in-between cycle execution, this event inhibits water feeding. Switch the sterilizer OFF and then ON again.
Check the external water supply system.
Check for water leaks from the sterilizer.
POSSIBLE CAUSE
Faulty MIN water level sensor in the clean water tank
SOLUTION
Call service. -
Lisa 17/22
The sterilization (PROCESS) phase of a sterilization cycle was longer than expected.POSSIBLE CAUSE
The chamber temperature dropped below the minimum threshold and the software performed a successful recovery.
SOLUTION
Wait for cycle completion. If the problem occurs frequently, call technical service. -
Warning about saving to the USB (HTML and SCL files).
The USB drive is disconnected or full. SOLUTION Check presence and condition of the USB drive. If the problem persists, contact an authorised W&H service partner.
-
Lisa 17/22
The cycle report printer does not work.POSSIBLE CAUSE
Printer not properly connected or not powered
SOLUTION
Check the data and the power connection to the printer. -
What should I do if I get a Warning about consumable replacement?
Order the requested consumable (door seal and HEPA/bacteriological filter) and replace it. See the chapter “Maintenance” in the Instructions for Use.
-
What is the maximum noise level of the Lara, Lara XL and Lexa Plus sterilizer?
The maximum noise level of Lara is 66.9 dB and the Lara XL/Lexa Plus are 70 dB.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
-
Lina (24)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Does my Lisa/Lina need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my Lisa/Lina?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
How do I clean the water tanks and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
How often should I de-scale my Thermoklenz?
Water hardness can differ depending of the region in which the unit is located. Carrying out a water hardness test will enable you to gauge how hard the incoming water is. The frequency of de-scaling cycles will depend on the water hardness and the number of cycles run per week. W&H recommends that at least one de-scaling cycle be run each week. More frequent de-scaling cycles should be run if the washer disinfector has a heavier workload or if the water is hard. Additional information relating to these tests and how to carry out these tests can be found on the Video Tutorial section of this web site.
-
Should I pre-wrap and how long can I store my instruments after sterilization?
Under current UK decontamination guidance, with a type B vacuum cycle, instruments should be pre-wrapped and once sterilized can be stored for up to 12 months. Wrapping should take place shortly after washing and disinfection. Under current UK decontamination guidance, with a type N cycle, instruments should not be pre-wrapped. Once sterilized, instruments can be immediately aseptically wrapped and stored for up to 12 months. Instruments should always be dry before they are placed in the purpose-designed packaging.
-
Lisa 17/22
No cycles are stored in the cycle history menuPOSSIBLE CAUSE
An electronic board was replaced by service.
SOLUTION
None. The memory of the old board cannot be restored. Save periodically the history on the USB pen drive. -
Lisa 17/22
The sterilizer enters into “Sleep mode” immediately after opening the chamber door.POSSIBLE CAUSE
The chamber door has not been opened after the previous cycle had finished and the “Sleep mode delay” has expired
SOLUTION
Press the SLEEP MODE button to exit”. -
Lisa 17/22
The sterilizer remains switched OFF.POSSIBLE CAUSE
The main switch or network circuit breaker is OFF
SOLUTION
Activate the main switch or network circuit breaker (ON).
POSSIBLE CAUSE
No voltage at the socket
SOLUTION
Check the electric circuit.
POSSIBLE CAUSE
The power cord is not connected properly
SOLUTION
Check and connect the power cord properly. -
Lisa 17/22
Water is leaking at the front of the sterilizerPOSSIBLE CAUSE
Leaks through the chamber door seal
SOLUTION
Clean or replace the door seal. Clean the chamber face side.
POSSIBLE CAUSE
Internal leak.
SOLUTIONS
Call technical service. -
Lisa 17/22
At the end of the cycle, there is residual water in the chamberPOSSIBLE CAUSE
Sterilizer not properly levelled
SOLUTION
Properly level the surface the sterilizer is placed on.
POSSIBLE CAUSE
Overloaded chamber
SOLUTION
Comply with the maximum load weight limits for each type of load. Always use the chamber rack for trays and cassettes.
POSSIBLE CAUSE
Chamber filter clogged
SOLUTION
Remove and clean the chamber filter.
POSSIBLE CAUSE
Chamber filter cap mispositioned
SOLUTION
Mount the chamber filter cap properly (see chapter MAINTENANCE)
POSSIBLE CAUSE
Load incorrectly placed
SOLUTION
Follow the recommendations as listed in ANNEX 2. -
Lisa 17/22
Corrosion or spots on instrumentsPOSSIBLE CAUSE
Tap water on instruments when placed in the sterilizer
SOLUTION
Ensure that instruments are dry before they are placed in the sterilizer.
POSSIBLE CAUSE
Use of water of poor quality or water containing chemical substances
SOLUTION
Drain both water tanks. Use water of good quality (see ANNEX 7).
POSSIBLE CAUSE
Organic or chemical residues on the instruments
SOLUTION
Clean, rinse and dry instruments before placing them in the sterilizer (see ANNEX 2).
POSSIBLE CAUSE
Chamber, trays, tray rack dirty
SOLUTION
Clean the chamber and wash the chamber furniture
POSSIBLE CAUSE
Contact between instruments of different materials
SOLUTION
Ensure that instruments of different materials do not touch (aluminum, carbon or stainless steel, etc.); place them on different trays or cassettes or pouch them (refer to ANNEX 2).
POSSIBLE CAUSE
Scale deposits on the chamber
SOLUTION
Clean the chamber and use water of good quality (refer to ANNEX 7). -
Lisa 17/22
When starting a cycle, the chamber door locks but re-opens immediately. The “Open the door” message appears.POSSIBLE CAUSE
Door seal not properly placed; seal sticking out
SOLUTION
Ensure that the door seal is correctly inserted around the whole circumference.
POSSIBLE CAUSE
Door jammed by external objects or by the load itself
SOLUTION
Remove any objects interfering with the chamber door. Check that the door does not push against the load or the chamber furniture. -
Lisa 17/22
Warning about programmed maintenance.POSSIBLE CAUSE
A component shall be replaced for the programmed maintenance of the sterilizer.
SOLUTION
Call service to order the requested component (door seal, dust filter, bacteriological filter...). See “Maintenance” chapter.. -
Lisa 17/22
When the sterilizer is connected to an automated water supply system: there is no clean water in the tank, but the automatic water filling does not fill the water.POSSIBLE CAUSE
Water fill system not connected
SOLUTION
Connect the water fill system to the sterilizer (see ANNEX 7 for water quality requirements).
POSSIBLE CAUSE
When the water fill system attempted to fill the tank, water was temporarily unavailable
SOLUTION
Since water tank filling is attempted only once in-between cycle execution, this event inhibits water feeding. Switch the sterilizer OFF and then ON again.
Check the external water supply system.
Check for water leaks from the sterilizer.
POSSIBLE CAUSE
Faulty MIN water level sensor in the clean water tank
SOLUTION
Call service. -
Warning about saving to the USB (HTML and SCL files).
The USB drive is disconnected or full. SOLUTION Check presence and condition of the USB drive. If the problem persists, contact an authorised W&H service partner.
-
Lisa 17/22
The cycle report printer does not work.POSSIBLE CAUSE
Printer not properly connected or not powered
SOLUTION
Check the data and the power connection to the printer. -
LINA PRO13-003
What is the maximum noise level of the sterilizer?The maximum noise level of 66.5 dB(A) guarantees a quiet working environment. -
What should I do if I get a Warning about consumable replacement?
Order the requested consumable (door seal and HEPA/bacteriological filter) and replace it. See the chapter “Maintenance” in the Instructions for Use.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
-
Lexa (9)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Does my Lisa/Lina need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my Lisa/Lina?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
How do I clean the water tanks and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
What should I do if I get a Warning about consumable replacement?
Order the requested consumable (door seal and HEPA/bacteriological filter) and replace it. See the chapter “Maintenance” in the Instructions for Use.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
-
Lyla (24)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Does my Lisa/Lina need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my Lisa/Lina?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
How do I clean the water tanks and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
Should I pre-wrap and how long can I store my instruments after sterilization?
Under current UK decontamination guidance, with a type B vacuum cycle, instruments should be pre-wrapped and once sterilized can be stored for up to 12 months. Wrapping should take place shortly after washing and disinfection. Under current UK decontamination guidance, with a type N cycle, instruments should not be pre-wrapped. Once sterilized, instruments can be immediately aseptically wrapped and stored for up to 12 months. Instruments should always be dry before they are placed in the purpose-designed packaging.
-
Lisa 17/22
No cycles are stored in the cycle history menuPOSSIBLE CAUSE
An electronic board was replaced by service.
SOLUTION
None. The memory of the old board cannot be restored. Save periodically the history on the USB pen drive. -
Lisa 17/22
The sterilizer enters into “Sleep mode” immediately after opening the chamber door.POSSIBLE CAUSE
The chamber door has not been opened after the previous cycle had finished and the “Sleep mode delay” has expired
SOLUTION
Press the SLEEP MODE button to exit”. -
Lisa 17/22
The sterilizer remains switched OFF.POSSIBLE CAUSE
The main switch or network circuit breaker is OFF
SOLUTION
Activate the main switch or network circuit breaker (ON).
POSSIBLE CAUSE
No voltage at the socket
SOLUTION
Check the electric circuit.
POSSIBLE CAUSE
The power cord is not connected properly
SOLUTION
Check and connect the power cord properly. -
Lisa 17/22
Water is leaking at the front of the sterilizerPOSSIBLE CAUSE
Leaks through the chamber door seal
SOLUTION
Clean or replace the door seal. Clean the chamber face side.
POSSIBLE CAUSE
Internal leak.
SOLUTIONS
Call technical service. -
Lisa 17/22
At the end of the cycle, there is residual water in the chamberPOSSIBLE CAUSE
Sterilizer not properly levelled
SOLUTION
Properly level the surface the sterilizer is placed on.
POSSIBLE CAUSE
Overloaded chamber
SOLUTION
Comply with the maximum load weight limits for each type of load. Always use the chamber rack for trays and cassettes.
POSSIBLE CAUSE
Chamber filter clogged
SOLUTION
Remove and clean the chamber filter.
POSSIBLE CAUSE
Chamber filter cap mispositioned
SOLUTION
Mount the chamber filter cap properly (see chapter MAINTENANCE)
POSSIBLE CAUSE
Load incorrectly placed
SOLUTION
Follow the recommendations as listed in ANNEX 2. -
Lisa 17/22
Corrosion or spots on instrumentsPOSSIBLE CAUSE
Tap water on instruments when placed in the sterilizer
SOLUTION
Ensure that instruments are dry before they are placed in the sterilizer.
POSSIBLE CAUSE
Use of water of poor quality or water containing chemical substances
SOLUTION
Drain both water tanks. Use water of good quality (see ANNEX 7).
POSSIBLE CAUSE
Organic or chemical residues on the instruments
SOLUTION
Clean, rinse and dry instruments before placing them in the sterilizer (see ANNEX 2).
POSSIBLE CAUSE
Chamber, trays, tray rack dirty
SOLUTION
Clean the chamber and wash the chamber furniture
POSSIBLE CAUSE
Contact between instruments of different materials
SOLUTION
Ensure that instruments of different materials do not touch (aluminum, carbon or stainless steel, etc.); place them on different trays or cassettes or pouch them (refer to ANNEX 2).
POSSIBLE CAUSE
Scale deposits on the chamber
SOLUTION
Clean the chamber and use water of good quality (refer to ANNEX 7). -
Lisa 17/22
When starting a cycle, the chamber door locks but re-opens immediately. The “Open the door” message appears.POSSIBLE CAUSE
Door seal not properly placed; seal sticking out
SOLUTION
Ensure that the door seal is correctly inserted around the whole circumference.
POSSIBLE CAUSE
Door jammed by external objects or by the load itself
SOLUTION
Remove any objects interfering with the chamber door. Check that the door does not push against the load or the chamber furniture. -
Lisa 17/22
Warning about programmed maintenance.POSSIBLE CAUSE
A component shall be replaced for the programmed maintenance of the sterilizer.
SOLUTION
Call service to order the requested component (door seal, dust filter, bacteriological filter...). See “Maintenance” chapter.. -
Lisa 17/22
When the sterilizer is connected to an automated water supply system: there is no clean water in the tank, but the automatic water filling does not fill the water.POSSIBLE CAUSE
Water fill system not connected
SOLUTION
Connect the water fill system to the sterilizer (see ANNEX 7 for water quality requirements).
POSSIBLE CAUSE
When the water fill system attempted to fill the tank, water was temporarily unavailable
SOLUTION
Since water tank filling is attempted only once in-between cycle execution, this event inhibits water feeding. Switch the sterilizer OFF and then ON again.
Check the external water supply system.
Check for water leaks from the sterilizer.
POSSIBLE CAUSE
Faulty MIN water level sensor in the clean water tank
SOLUTION
Call service. -
Lisa 17/22
The sterilization (PROCESS) phase of a sterilization cycle was longer than expected.POSSIBLE CAUSE
The chamber temperature dropped below the minimum threshold and the software performed a successful recovery.
SOLUTION
Wait for cycle completion. If the problem occurs frequently, call technical service. -
Warning about saving to the USB (HTML and SCL files).
The USB drive is disconnected or full. SOLUTION Check presence and condition of the USB drive. If the problem persists, contact an authorised W&H service partner.
-
Lisa 17/22
The cycle report printer does not work.POSSIBLE CAUSE
Printer not properly connected or not powered
SOLUTION
Check the data and the power connection to the printer. -
What should I do if I get a Warning about consumable replacement?
Order the requested consumable (door seal and HEPA/bacteriological filter) and replace it. See the chapter “Maintenance” in the Instructions for Use.
-
Which is the maximum noise level of Lyla?
The max. noise level of Lyla is 65.5 dB.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
-
Lara XL (10)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
How do I clean the water tanks and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
Lisa 17/22
Warning about programmed maintenance.POSSIBLE CAUSE
A component shall be replaced for the programmed maintenance of the sterilizer.
SOLUTION
Call service to order the requested component (door seal, dust filter, bacteriological filter...). See “Maintenance” chapter.. -
Lisa 17/22
When the sterilizer is connected to an automated water supply system: there is no clean water in the tank, but the automatic water filling does not fill the water.POSSIBLE CAUSE
Water fill system not connected
SOLUTION
Connect the water fill system to the sterilizer (see ANNEX 7 for water quality requirements).
POSSIBLE CAUSE
When the water fill system attempted to fill the tank, water was temporarily unavailable
SOLUTION
Since water tank filling is attempted only once in-between cycle execution, this event inhibits water feeding. Switch the sterilizer OFF and then ON again.
Check the external water supply system.
Check for water leaks from the sterilizer.
POSSIBLE CAUSE
Faulty MIN water level sensor in the clean water tank
SOLUTION
Call service. -
Warning about saving to the USB (HTML and SCL files).
The USB drive is disconnected or full. SOLUTION Check presence and condition of the USB drive. If the problem persists, contact an authorised W&H service partner.
-
Lisa 17/22
The cycle report printer does not work.POSSIBLE CAUSE
Printer not properly connected or not powered
SOLUTION
Check the data and the power connection to the printer. -
What should I do if I get a Warning about consumable replacement?
Order the requested consumable (door seal and HEPA/bacteriological filter) and replace it. See the chapter “Maintenance” in the Instructions for Use.
-
What is the maximum noise level of the Lara, Lara XL and Lexa Plus sterilizer?
The maximum noise level of Lara is 66.9 dB and the Lara XL/Lexa Plus are 70 dB.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
-
Lisa (26)
-
Do I need to carry out periodic testing on my W&H Sterilizer?
All W&H sterilizers features test cycles (B&D/Helix test, Vacuum test). Compulsory and frequency of testing are regulated by local/national guidelines.
-
What is validation?
Validation is a series of tests carried out with the use of independent specialist test equipment, as is necessary to demonstrate that the physical conditions required for sterilization, thermal disinfection and/or cleaning (i.e. temperature, pressure, time) are achieved.
-
Do I need to document the results of all periodic tests?
Yes, all tests performed by the operator or user should be recorded in a logbook with the date and signature of the individual how carried out the test. Every Sterilizer and every thermo washer disinfector should have a logbook (file) in which test details pertaining to the lifecycle of the equipment (from purchase - disposal) are recorded. A logbook is provided as standard with the Premium Careplus Service Contract from W&H, or as an optional extra with the Premium Care Service Contract.
-
How long should I Keep periodic test records?
Under current national decontamination guildance, all audit documents should be stored for at least two years and they should not be removed from the premises or destroyed.
-
Does my Lisa/Lina need to be commissioned from new?
Yes, validation is needed for new equipment at installation and annually thereafter. It will also be necessary to validate equipment after any major repairs have been carried out.
-
Who should I use to carry out validation and maintenance on my Lisa/Lina?
All validation and maintenance should be undertaken by the Competent Person (Decontamination) or Competent Person (maintenance).
-
How do I change consumable components on my sterilizer?
Consumable replacement instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
How do I clean the water tanks and what products should I use?
Water tank cleaning instructions are provided in the Instructions for Use, chapter “Maintenance”. In addition, a video description is available: • in the sterilizer, in the maintenance section of the menu; • in the Consumables & Accessories and Video Tutorial section of this website.
-
What water quality should I use with my Lisa/Lina?
The water quality for sterilizers is internationally specified by standards and directives. The reasons for this are that your sterilizer can be seriously damaged by it or the sterilization can be jeopardised. Consequently, only water with conductivity of less than 10 µS/cm in North America and 15 µS/cm for the rest of the world should be used for your W&H sterilizer. The Water treatment devices Multidem and Osmo are especially designed to prepare suitable water (see chapter “Water quality” in the Instructions for Use).
-
How often should I de-scale my Thermoklenz?
Water hardness can differ depending of the region in which the unit is located. Carrying out a water hardness test will enable you to gauge how hard the incoming water is. The frequency of de-scaling cycles will depend on the water hardness and the number of cycles run per week. W&H recommends that at least one de-scaling cycle be run each week. More frequent de-scaling cycles should be run if the washer disinfector has a heavier workload or if the water is hard. Additional information relating to these tests and how to carry out these tests can be found on the Video Tutorial section of this web site.
-
Should I pre-wrap and how long can I store my instruments after sterilization?
Under current UK decontamination guidance, with a type B vacuum cycle, instruments should be pre-wrapped and once sterilized can be stored for up to 12 months. Wrapping should take place shortly after washing and disinfection. Under current UK decontamination guidance, with a type N cycle, instruments should not be pre-wrapped. Once sterilized, instruments can be immediately aseptically wrapped and stored for up to 12 months. Instruments should always be dry before they are placed in the purpose-designed packaging.
-
Lisa 17/22
No cycles are stored in the cycle history menuPOSSIBLE CAUSE
An electronic board was replaced by service.
SOLUTION
None. The memory of the old board cannot be restored. Save periodically the history on the USB pen drive. -
Lisa 17/22
The sterilizer enters into “Sleep mode” immediately after opening the chamber door.POSSIBLE CAUSE
The chamber door has not been opened after the previous cycle had finished and the “Sleep mode delay” has expired
SOLUTION
Press the SLEEP MODE button to exit”. -
Lisa 17/22
The sterilizer remains switched OFF.POSSIBLE CAUSE
The main switch or network circuit breaker is OFF
SOLUTION
Activate the main switch or network circuit breaker (ON).
POSSIBLE CAUSE
No voltage at the socket
SOLUTION
Check the electric circuit.
POSSIBLE CAUSE
The power cord is not connected properly
SOLUTION
Check and connect the power cord properly. -
Lisa 17/22
Water is leaking at the front of the sterilizerPOSSIBLE CAUSE
Leaks through the chamber door seal
SOLUTION
Clean or replace the door seal. Clean the chamber face side.
POSSIBLE CAUSE
Internal leak.
SOLUTIONS
Call technical service. -
Lisa 17/22
At the end of the cycle, there is residual water in the chamberPOSSIBLE CAUSE
Sterilizer not properly levelled
SOLUTION
Properly level the surface the sterilizer is placed on.
POSSIBLE CAUSE
Overloaded chamber
SOLUTION
Comply with the maximum load weight limits for each type of load. Always use the chamber rack for trays and cassettes.
POSSIBLE CAUSE
Chamber filter clogged
SOLUTION
Remove and clean the chamber filter.
POSSIBLE CAUSE
Chamber filter cap mispositioned
SOLUTION
Mount the chamber filter cap properly (see chapter MAINTENANCE)
POSSIBLE CAUSE
Load incorrectly placed
SOLUTION
Follow the recommendations as listed in ANNEX 2. -
Lisa 17/22
Corrosion or spots on instrumentsPOSSIBLE CAUSE
Tap water on instruments when placed in the sterilizer
SOLUTION
Ensure that instruments are dry before they are placed in the sterilizer.
POSSIBLE CAUSE
Use of water of poor quality or water containing chemical substances
SOLUTION
Drain both water tanks. Use water of good quality (see ANNEX 7).
POSSIBLE CAUSE
Organic or chemical residues on the instruments
SOLUTION
Clean, rinse and dry instruments before placing them in the sterilizer (see ANNEX 2).
POSSIBLE CAUSE
Chamber, trays, tray rack dirty
SOLUTION
Clean the chamber and wash the chamber furniture
POSSIBLE CAUSE
Contact between instruments of different materials
SOLUTION
Ensure that instruments of different materials do not touch (aluminum, carbon or stainless steel, etc.); place them on different trays or cassettes or pouch them (refer to ANNEX 2).
POSSIBLE CAUSE
Scale deposits on the chamber
SOLUTION
Clean the chamber and use water of good quality (refer to ANNEX 7). -
Lisa 17/22
When starting a cycle, the chamber door locks but re-opens immediately. The “Open the door” message appears.POSSIBLE CAUSE
Door seal not properly placed; seal sticking out
SOLUTION
Ensure that the door seal is correctly inserted around the whole circumference.
POSSIBLE CAUSE
Door jammed by external objects or by the load itself
SOLUTION
Remove any objects interfering with the chamber door. Check that the door does not push against the load or the chamber furniture. -
Lisa 17/22
Warning about programmed maintenance.POSSIBLE CAUSE
A component shall be replaced for the programmed maintenance of the sterilizer.
SOLUTION
Call service to order the requested component (door seal, dust filter, bacteriological filter...). See “Maintenance” chapter.. -
Lisa 17/22
When the sterilizer is connected to an automated water supply system: there is no clean water in the tank, but the automatic water filling does not fill the water.POSSIBLE CAUSE
Water fill system not connected
SOLUTION
Connect the water fill system to the sterilizer (see ANNEX 7 for water quality requirements).
POSSIBLE CAUSE
When the water fill system attempted to fill the tank, water was temporarily unavailable
SOLUTION
Since water tank filling is attempted only once in-between cycle execution, this event inhibits water feeding. Switch the sterilizer OFF and then ON again.
Check the external water supply system.
Check for water leaks from the sterilizer.
POSSIBLE CAUSE
Faulty MIN water level sensor in the clean water tank
SOLUTION
Call service. -
Lisa 17/22
The sterilization (PROCESS) phase of a sterilization cycle was longer than expected.POSSIBLE CAUSE
The chamber temperature dropped below the minimum threshold and the software performed a successful recovery.
SOLUTION
Wait for cycle completion. If the problem occurs frequently, call technical service. -
Warning about saving to the USB (HTML and SCL files).
The USB drive is disconnected or full. SOLUTION Check presence and condition of the USB drive. If the problem persists, contact an authorised W&H service partner.
-
Lisa 17/22
The cycle report printer does not work.POSSIBLE CAUSE
Printer not properly connected or not powered
SOLUTION
Check the data and the power connection to the printer. -
Lisa 17/22
What is the maximum noise level of the sterilizer?The maximum running noise level of 58.5 dB(A)ensures a quiet, comfortable working environment. -
What should I do if I get a Warning about consumable replacement?
Order the requested consumable (door seal and HEPA/bacteriological filter) and replace it. See the chapter “Maintenance” in the Instructions for Use.
-
What is the maximum noise level of the Lisa sterilizer?
The max. noise level of Lisa 2019 is 64.3 dB.
-
Do I need to carry out periodic testing on my W&H Sterilizer?
 Air Motor
Air Motor

-
Does the air motor need to be lubricated?
Yes, the air motor must be lubricated as described in the "Hygiene and care" section of the instructions for use.

