Ultrasound-activated mechanical biofilm management
by Prof. Dirk Ziebolz and Barbara Kampfmann
First published: Implantologie Journal 3/2019
Continued maintenance of peri-implant health and the associated, targeted prevention of peri-implant disease are essential to ensuring long-term implant success.(1-3) Accordingly, the ongoing professional and prevention-oriented care of implant patients in the scope of supportive implant therapy (SIT) is of particular importance.(3-7) The case described here illustrates the possibilities for employing a piezoelectric ultrasonic scaler in the scope of SIT for the efficient cleaning of tooth and implant surfaces.
On the one hand, it is important in SIT that the patient practices good oral hygiene at home. For example, the patient should be regularly encouraged to employ appropriate oral hygiene measures and also instructed in their correct performance.(2) On the other hand, professional dental care including regular clinical diagnostics and implant cleaning performed by the dental team is also essential.(2)
Regular mechanical cleaning of the implant and tooth surfaces is a focus of implant aftercare. In this respect, it is possible to draw on the largely tried-and-tested procedures employed in supportive periodontal therapy (SPT). The use of standard curettes and scalers as well as oscillating sonic and ultrasonic instruments, either individually or in combination, has established itself as the gold standard for mechanical biofilm removal.(8) It therefore seems obvious that the use of such instruments could also prove safe and effective in implant aftercare. Accordingly, there is a wide range of different (implant-)specific instruments and systems available for the mechanical cleaning of the implants: for example, hand instruments (scalers, curettes), oscillating sonic and ultrasonic instruments, air-powder-water jet devices and laser-based systems.(2,9-12)
An array of special carbon, titanium and plastic curettes and scalers are available for the mechanical cleaning of implant surfaces by hand.(13-15) Overall, the efficacy of the biofilm removal with the available hand instruments is predominantly assessed as ineffective.(11) Whilst plastic curettes in particular have only a minimal detrimental effect on the implant surface and afford patients a high degree of comfort, their poor efficiency with regard to biofilm removal and their incapacity to remove calculus and subgingival concretions contraindicate their use.(16,17) Today, the use of carbon and titanium curettes is viewed as being more efficient, with the result that, if at all, these can be recommended for use as hand instruments.(11)
Alongside the use of manual instruments, the increased use of air-powered sonic instruments and piezoelectric/magnetostrictive ultrasonic instruments (scalers) for the cleaning of tooth and implant surfaces has also proven itself.(11) Modified working tips (e.g. on a carbon or PEEK basis) are employed so as to avoid possible damage and irritation when cleaning the abutment and superstructure surface. The cleaning efficiency is reported to be good.(11) The use of corresponding sonic/ultrasonic systems for implant cleaning individually or in combination with hand instruments can thus be viewed as widely accepted by the scientific dental community.
However, all in all, it must still be considered that it has yet to be definitively clarified which of the procedures outlined above, individually or in combination, can be employed most effectively for the prevention of peri-implant disease. In the scope of professional implant cleaning, the combined use of hand and sonic/ultrasonic instruments, possibly complemented with air-powder-water jet systems, appears recommendable.(18) When employed correctly, it is possible to attain and maintain a stable peri-implant situation. It is also important not to neglect the subsequent polishing for smoothing of all implant and tooth surfaces. No additional clinical benefit is to be expected from the application of chemical substances.(2,18) According to our current understanding, the use of ultrasonic systems individually or in combination with special hand instruments promises an effective strategy for the prevention of peri-implant mucositis and peri-implantitis. The case described here illustrates the possibilities for employing a piezoelectric ultrasonic scaler for efficient cleaning of tooth and implant surfaces.
Description of patient case
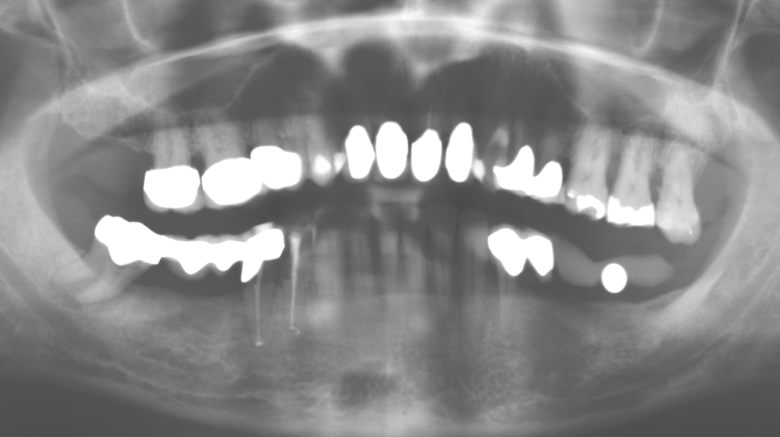
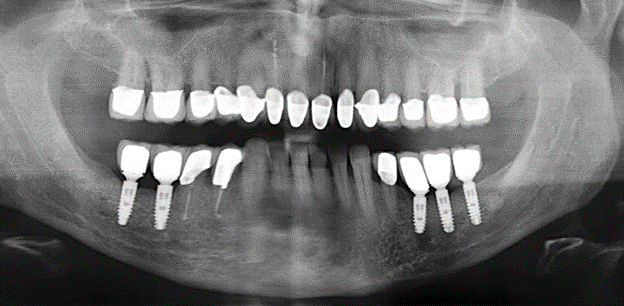
A 52-year-old patient presented in our clinic for the first time in 2004 following tooth loss in the third quadrant, expressing a desire for a new prosthetic restoration. Periodontal and radiological diagnostics revealed the need for extensive periodontological treatment. In addition, teeth 48, 28 and 27 were attributed a very poor prognosis and were subsequently extracted (Fig. 1). Following the successfully completed, systematic periodontological treatment, a fixed dental implant was inserted with the introduction of five implants in tooth regions 35, 36, 37, 46 and 47. Prosthetic treatment of the natural teeth was effected with veneered zirconium dioxide ceramic crowns; the implants were composed of two-piece, individual zirconium dioxide abutments and similarly veneered crowns made of a zirconium dioxide ceramic (Cercon base colored, Dentsply Sirona Lab). Definitive insertion of the prosthetic restoration occurred in 2005.
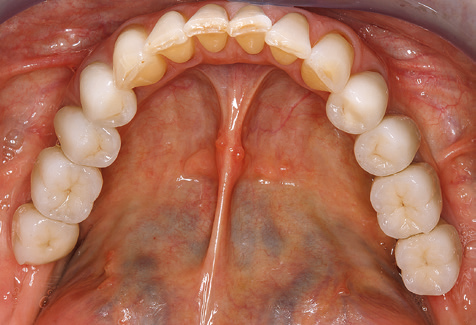
Due to the presence of periodontal disease, SPT was performed every three months in the first years following the insertion. The patient demonstrated a high degree of motivation and good compliance. The pocket depths recorded annually revealed a stable periodontal situation with a BOP index of below five per cent. On the basis of the stable periodontal situation and good cooperation on the patient’s part, the recall interval was extended to every six months as of the sixth year of the prosthetic function phase. Following the change in the recall interval, the respective annual documentation of the periodontal status continued to reveal a stable periodontal situation with no increase in the pocket depths and a BOP index below five per cent (Fig. 2a and b).
The ten-year check-up revealed no indications of advancing clinical attachment loss or peri-implant bone substance loss (Fig. 3).
The patient continues to visit the clinic every six months for SIT. In the following, the patient is taken as an example for demonstrating the individual working steps in a structured SPT session as it has been performed with barely any modifications over the last 12 years. Of course, some new materials and devices have been integrated into the concept over the years. This clinical case report presents the current material and device concept.
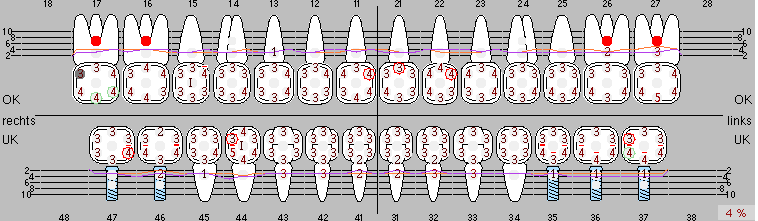
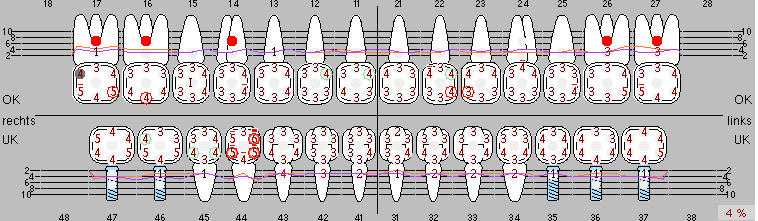
Fig. 2: The pocket depths recorded annually with six-monthly SPT display no increasing trend and a BOP index of below 5 per cent with a largely inflammation-free periodontal situation. a) PERIO status in 2011 (after five years with implants). b) PERIO status in 2016 (after ten years with implants).
The current working concept for SPT
Updating the patient’s medical history is an important aspect of SPT and should occur at least once per year. It helps the dental team to identify and document any new risk factors. Especially when a patient is treated over many years, it is important to establish whether patient-specific and general health risk factors have changed. This primarily concerns a heightened risk as a result of diabetes, but other general conditions (cardiovascular disease and neoplasia) can also produce a modified risk profile as a result of the treatment performed and medication administered. Accordingly, updating the medical history as part of SPT is very important, as a modified risk profile may trigger the need to adapt the treatment interval. In the next step, it is important to afford the diagnostics due attention. Whilst instruments are a central aspect of SPT, findings and their documentation must never be neglected. The periodontological findings are essential for a good diagnosis; increases in the pocket depths and the BOP index are clear indicators of advancing periodontal and peri-implant disease. As such, the team should not shy away from probing implants too, with the aim of gathering the requisite data. At the same time, it is important to use periodontal probes with millimetre markings. Metallic probes have already been used for determining pocket depths around natural teeth for decades. In the case of implants, the challenge of recording correct and reproducible pockets depths is even greater. As the discrepancy between the implant diameter and the contour of the superstructure regularly results in overcontouring of the superstructure, flexible probes which still feature millimetre markings are a sensible solution for measuring pocket depths around implants (e.g., Colorvue Kit PCV11KIT6, HuFriedy; Fig. 4).
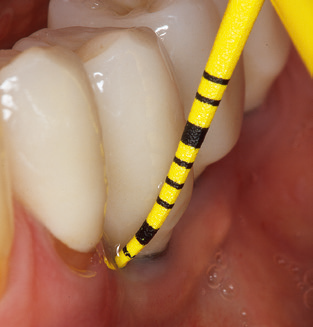
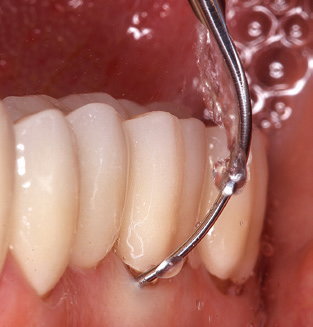
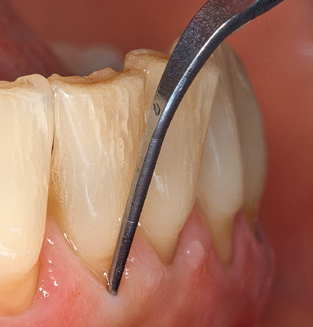
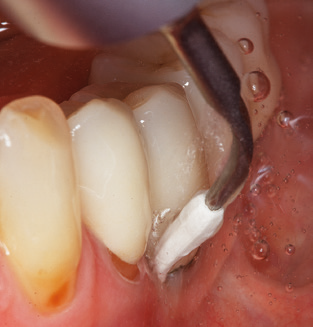
In patients without special risk factors, the complete PERIO status should be documented at least once per year. In cases with multiple risk factors (diabetes, smoking, etc.), more regular monitoring (every six months) can prove prudent.
In the scope of the diagnostics, it is of course relevant to consider at what stage x-rays become necessary. Nowadays, X-ray diagnostics should only be employed when the risks indicate their necessity. Specifically, this means that x-rays are only taken in order to verify clinical findings which point towards progression of the disease (increased pocket depths or BOP index).
Of course, the use of instruments for mechanical removal of the biofilm is a central component of SPT and thus of primary significance. Consequently, the SPT workflow comprises both supragingival and subgingival cleaning. In our concept, a combination of hand instruments and machine cleaning has proven advantageous. A number of options are available for the mechanical procedures: sonic devices, ultrasonic devices and powder jet devices.
Although the fundamental principle of the ultrasonic devices remains, recent years have seen a whole host of further developments, resulting in an increase in efficiency, patient comfort and safety. These innovations are all present in the ultrasonic device (Tigon+, W&H Dentalwerk Bürmoos GmbH) currently employed by the authors, for example.
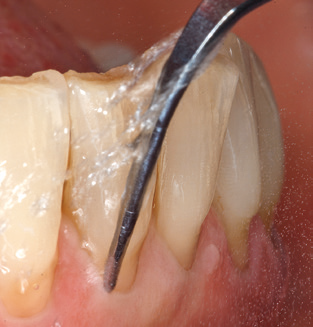
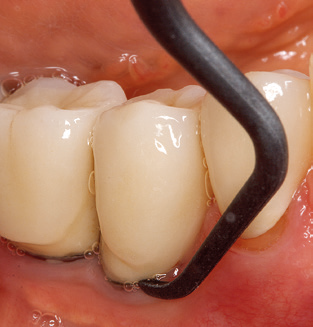
Good illumination of the working field facilitates the process considerably. The system used by the authors achieves this thanks to a 5x LED ring integrated in the handpiece. Naturally, a range of working tips for different indications is also offered. A straight, universally employable tip is the basic instrument required for machine cleaning of natural teeth (Fig. 5a and b). Curved tips, which allow access to exposed furcations, are also available for hard-to-reach areas in the posterior region (Fig. 6).
Of course, working tips for the cleaning of implant surfaces are also indispensable for SPT in patients fitted with implants. The implant cleaning attachment on the system used here is characterised by its tapered, hexagonal design. This design allows light, atraumatic penetration of the peri-implant pocket and displays a good cleaning performance (Fig. 7).
Patient feedback gathered has revealed that the system also offers two benefits which increase patient comfort: namely, the preheating of the rinsing fluid, which avoids irritation when cleaning sensitive tooth surfaces, and the system’s Smooth mode. In this mode, the system’s power decreases the harder the tip is pressed against the tooth. Both aspects make the treatment more pleasant for patients than with other ultrasonic systems used in the past.
A third and exceedingly relevant aspect in favour of the use of this system is the fact that it is also approved for use in patients fitted with pacemakers. The use of ultrasonic instruments in these patients was contraindicated in the past – now there is a system available which is truly universally applicable.
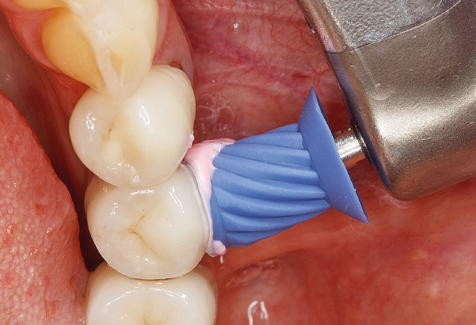
Following machine cleaning of the tooth and implant surfaces, the surfaces of the natural teeth are cleaned manually using standard hand instruments. When performing manual cleaning, particular attention must be given to maintaining the correct angle of application, appropriate sharpness, good support and working with the curette from apical to coronal. Either titanium or carbon curettes should be used for post-cleaning of the implant structures (Fig. 8). In addition to the use of ultrasonic devices, power jet devices can also be used in conservative dentistry. However, it must be taken into consideration that these procedures are not suitable for removing hard deposits and thus they cannot replace the use of hand instruments and ultrasonic instruments completely. In all cases, cleaning is followed by mechanical polishing of the accessible tooth and implant surfaces with polishing cups and polishing compounds (Fig. 9).
If necessary, the patient is instructed again in the use of suitable aids for proper oral hygiene at home (Fig. 10).
To date, the benefit of additional application of antibacterial agents in the scope of SPT has yet to be sufficiently clinically proven, and this is therefore not recommended as a component of basic treatment. The focus here is on mechanical removal of the biofilm.
An SPT session employing the workflow outlined here generally takes around 60 minutes. A practical way to finish is to arrange the next SPT appointment.
Summary
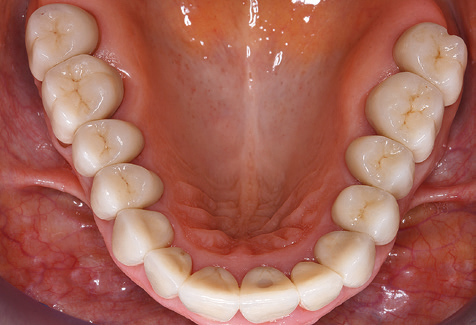
Standardised and regular risk-adapted care in the scope of SPT is the key to treatment success for the clinical long-term success in periodontically compromised patients. This is particularly true for patients fitted with implants following successfully completed periodontal treatment (Fig. 11a and b).
Before initiating the prosthetic treatment, it is advisable to inform the patient of the time and financial costs associated with the SPT (two to four SPT sessions per year for the rest of his/her life). This contributes significantly to ensuring the patient’s compliance. In addition, the practice is required to provide the rooms and staff necessary for the care of the periodontically treated patients.
Author’s contact information
Prof. Dr. Dirk Ziebolz, M.Sc.
University Hospital Leipzig, Department of Conservative Dentistry and Periodontology
Liebigstrasse 10-14, 04103 Leipzig, Germany
dirk.ziebolz@medizin.uni-leipzig.de
www.zahnerhaltung.uniklinikum-leipzig.de
DH Barbara Kampfmann
Praxisklinik für Zahnmedizin
Priv.-Doz. Dr. S. Rinke, M.Sc., M.Sc.,Dr. M. Jablonski, H. Ziebolz & Kollegen
Geleitstraße 68, 63456 Hanau, Germany
hanau@ihr-laecheln.com
www.ihr-laecheln.com
more info

Literature
- Heitz-Mayfield LJ: Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 2008;35 (Suppl 8):292-304.
- Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, et al.: Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol 2015; 42 (Suppl 16):152-157.
- Monje A, Aranda L, Diaz KT, Alarcón MA, Bagramian RA, Wang HL, et al.: Impact of Maintenance Therapy for the Prevention of Peri-implant Diseases: A Systematic Review and Meta-analysis. J Det Res 2016;43:323-334.
- Pjetursson BE, Helbling C, Weber HP, Matuliene G, Salvi GE, Brägger U, et al.: Peri-implantitis susceptibility as it relates to periodontal therapy and supportive care. Clin Oral Implants Res 2012;23:888-894.
- Roccuzzo M, De Angelis N, Bonino L, Aglietta M: Ten-year results of a three-arm prospective cohort study on implants in periodontally compromised patients. Part 1: Implant loss and radiographic bone loss. Clin Oral Implants Res 2010;21:490-496.
- Roccuzzo M, Bonino F, Aglietta M, Dalmasso P: Ten-year results of a three-arms prospective cohort study on implants in periodontally compromised patients. Part 2: Clinical results. Clin Oral Implants Res 2012;23:389-395.
- Roccuzzo M, Bonino L, Dalmasso P, Aglietta M: Long-term results of a three-arms prospective cohort study on implants in periodontally compromised patients. Part 3: 10-year data around sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res 2014;25:1105-1112.
- Graetz C, Bräuning A, Plaumann A, Springer C, Kahl M, Dörfer CE. Antiinfektiöse Therapie - Instrumente zur Wurzeloberflächenbearbeitung im Fokus. Parodontologie 2016; 27:165-183
- Renvert S, Roos-Jansåker AM, Claffey N: Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 2008; 35 (Suppl 8):305-315.
- Renvert S, Polyzois I, Persson GR: Treatment modalities for peri-implant mucositis and peri-implantitis. Am J Dent 2013;26:313-318
- Louropoulou A, Slot DE, Weijden F: The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Implants Res 2014;25:1149-1160.
- Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000 2014;66:255-273.
- Mann M, Parmar D, Walmsley AD, Lea SC: Effect of plastic-covered ultrasonic scalers on titanium implant surfaces. Clin Oral Impl Res 2012;23:76-82.
- Mengel R, Buns CE, Mengel C, Flores-de-Jacoby L: An in vitro study of the treatment of implant surfaces with different instruments. Int J Oral Maxillofac Implants 1998;13:91-96.
- Mengel R, Meer C, Flores-de-Jacoby L: The treatment of uncoated and titanium nitride-coated abutments with different instruments. Int J Oral Maxillofac Implants 2004;19:232-238.
- Fox SC, Moriarty JD, Kusy RP: The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol 1990;61:4854-90.
- Dmytryk JJ, Fox SC, Moriarty JD: The effects of scaling titanium implant surfaces with metal and plastic instruments on cell attachment. J Periodontol 1990;61:491-496.
- Ziebolz D, Klipp S, Schmalz G, Schmickler J, Rinke S, Kottmann T, et al.: Comparison of different maintenance strategies within supportive implant therapy for prevention of peri-implant inflammation during the first year after implant restauration – a randomized, practice-based multicenter study of a dental hygienist setting. Am J Dent 2017;30:190-196.


comments